In a joint announcement, both NASA (National Aeronautics and Space Administration) and NOAA (National Oceanic and Atmospheric Administration) have released findings from the Aura satellite which shows that from September 21st through the 30th of 2006, the Antarctic ozone hole was the largest ever recorded.
“From September 21 to 30, the average area of the ozone hole was the largest ever observed, at 10.6 million square miles,” said Paul Newman, atmospheric scientist at NASA’s Goddard Space Flight Center, Greenbelt, Md. If the stratospheric weather conditions had been normal, the ozone hole would be expected to reach a size of about 8.9 to 9.3 million square miles, about the surface area of North America.
Ozone, or O3, is simply three oxygen atoms formed into a single triatomic molecule. It is far less stable than O2, and as such is present in fairly low concentrations throughout the atmosphere. However, in the stratosphere, ozone concentrations act to filter out high energy ultraviolet light from the sun. Known as the ozone layer, without this filtering mechanism, sufficient quantities of ultraviolet light will damage skin by sunburn, and can even lead to a variety of skin cancers. A complete destruction of the earth’s ozone layer would be an environmental disaster, likely leaving the planet uninhabitable for most plant and animal life.
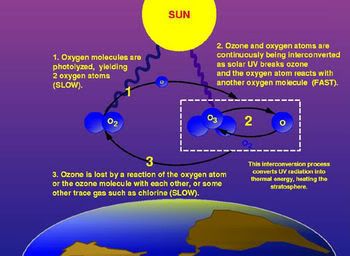 The story of ozone begins in the 1930s, when English physicist Sidney Chapman first formulated a theory of atmospheric ozone creation and destruction known as the Ozone Cycle. However, instrument measurements of the actual atmospheric ozone content showed a significant discrepancy between the ozone density as recorded compared to calculations done using his theory. Atmospheric physicists were perplexed until the early 1970s when three atmospheric physicists, Professors Paul Crutzen, Mario Molina, and F. Sherwood Rowland, each began to explore this discrepancy from different angles.
The story of ozone begins in the 1930s, when English physicist Sidney Chapman first formulated a theory of atmospheric ozone creation and destruction known as the Ozone Cycle. However, instrument measurements of the actual atmospheric ozone content showed a significant discrepancy between the ozone density as recorded compared to calculations done using his theory. Atmospheric physicists were perplexed until the early 1970s when three atmospheric physicists, Professors Paul Crutzen, Mario Molina, and F. Sherwood Rowland, each began to explore this discrepancy from different angles.
Professor Crutzen, in 1970, untangled a link between certain soil microorganisms and ozone destruction, determining that these bacteria release nitrogen oxides which react as a catalytist to destroy ozone. In 1974 professors Molina and Sherwood then published a study in Nature showing a connection between chlorofluorocarbons (CFCs), chemicals in widespread use throughout industry as a refrigerant, in plastics and insulation production, and — most well known — as a supposedly inert gas to pressurize spray cans, could break down and destroy ozone in the stratosphere.
Their work showed that CFCs, while inert in the lower atmosphere, could float up to the stratosphere, then react strongly with ambient ultraviolet light to break down into chlorine, which would then act to break down ozone. Newly created instruments then detected measurable global atmospheric CFC content throughout the world. While industry leaders downplayed the situation, by the late 1970s near worldwide concern of the use of CFCs led some to suggest a worldwide ban on CFC production. In 1996 the three won the Nobel Prize in Chemistry for their work.
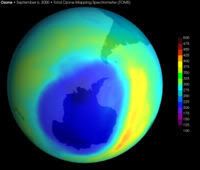 By 1983 the predictions of declining atmospheric ozone were alarmingly confirmed when Dr. Joseph Farman (BBC interview of Dr. Farman on the discovery), a British Antarctic survey scientist, discovered a curious ozone hole in the stratosphere while collecting atmospheric data in the Antarctic. At first he and his colleges “… doubted the validity of their own measurements …” however their work was “… was quickly confirmed by measurements from satellites and from other Antarctic research stations.” (7th paragraph) Due to graphic imagery from NASA and NOAA weather satellites, the story soon made its way to the popular press — as shown by this old 1987 Time Magazine article. By 1985 the ozone hole was widely understood to be growing in size for reasons unknown, and was considered a serious environmental threat worth serious inquiry.
By 1983 the predictions of declining atmospheric ozone were alarmingly confirmed when Dr. Joseph Farman (BBC interview of Dr. Farman on the discovery), a British Antarctic survey scientist, discovered a curious ozone hole in the stratosphere while collecting atmospheric data in the Antarctic. At first he and his colleges “… doubted the validity of their own measurements …” however their work was “… was quickly confirmed by measurements from satellites and from other Antarctic research stations.” (7th paragraph) Due to graphic imagery from NASA and NOAA weather satellites, the story soon made its way to the popular press — as shown by this old 1987 Time Magazine article. By 1985 the ozone hole was widely understood to be growing in size for reasons unknown, and was considered a serious environmental threat worth serious inquiry.
In 1986, Susan Soloman, a senior scientist with NOAA, along with several others, proposed the first model to explain why ozone above Antarctica might decline to nearly nothing in a yearly cycle of ozone accumulation and destruction, while remaining of fairly consistent (if declining) density in the stratosphere throughout the rest of the world. She suggested it was the extreme cold of Antarctic atmospheric conditions, due to higher than normal sulfuric acid concentrations in stratospheric clouds, that reacted with CFCs to increase ozone destruction beyond the already understood destructive component of ultraviolet light. (11th paragraph)
Due to longstanding concerns about CFCs, and the severity of the data collected, action was swift. In 1987 the Montreal Protocol, an international treaty banning CFC production, was opened for signature and finally ratified by the United States in 1989. Since then most nations worldwide have followed suit, due to the alarming dangers of continued ozone depletion.
Weather satellites of varying capacity have been tracking the situation ever since, the latest of which is known as Aura. It creates daily maps of ozone density, providing a nice detailed image of ozone density over time. It is this satellite, and it’s Ozone Monitoring Instrument (OMI), which collected the data from September 21st to the 30th. What it found is alarming. According to the press release, the instrument recorded that the hole itself comprised 10.6 million square miles, compared to an expected 8.9 – 9.3 million miles. Further, on October 9th, balloon and satellite had “…plunged to 93 DU (Dobson Units) from approximately 300 DU in mid-July…” (5th paragraph). The Dobson Unit is a measure of atmospheric ozone concentration.
David Hoffman was quoted in the press release:
“These numbers mean the ozone is virtually gone in this layer of the atmosphere,” said David Hofmann, director of the Global Monitoring Division at the NOAA Earth System Research Laboratory. “The depleted layer has an unusual vertical extent this year, so it appears that the 2006 ozone hole will go down as a record-setter.”
Fortunately, scientists do believe the ozone hole is nearing the apex of its increasing size. Due to reductions in worldwide CFC gas production, scientists believe it “…is estimated to annually very slowly decrease in area by about 0.1 to 0.2 percent for the next five to 10 years.” (11th paragraph) But the long half-life of ozone depleting chemicals can last for as many as 40 years. Meaning constant satellite vigilence of the ozone hole may be necessary for decades, perhaps even centuries, to come.
—–
Images courtesy Wikipedia and under a Creative Commons copyright license.
Text Copyright ©2006 J. Maynard Gelinas.
Updates and archive at daduh.org.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 2.5 License.




